The city of Padua has a dualistic approach to health. It was, and still is, a place of pilgrimage – generations have flocked to the Basilica of Saint Anthony since the 13th century, seeking healing for their ills. At the same time, it has a storied medical history: some of the earliest European hospitals have been built in Padua. Here, Giovanni Battista da Monte (1489 – 1551), professor of medicine at the University, first formalised the concept of “clinic”, as in, a place where physicians visited patients at their beds.
The city is home to the Padova Neuroscience Center (PNC), research centre of the University of Padua. Its founding director was Maurizio Corbetta, who is also a Professor of Neurology in the Department of Neuroscience and Director of the Neurology Clinic. Corbetta is also leader of the Work Package 2 of EBRAINS 2.0, which is devoted to human clinical maps and connectomes.
EBRAINS 2.0 is an EU-funded project to further develop the EBRAINS Research Infrastructure technologies to the scientific community.
European collaboration is in full swing for Corbetta: In July he will present EBRAINS in a lecture at the European Academy of Neurology (EAN) congress in Helsinki. EAN is Europe‘s largest organisation in neurology, representing over 45,000 European neurologists. Corbetta’s home institution, University of Padua, is joining the EBRAINS community as an associated member and he is active in EBRAINS Italy, the Italian national Node of EBRAINS. In September 2024 he will organize the BigBrain international workshop in Padua, a large EU-Canada collaboration in brain mapping and AI which is powered by EBRAINS.
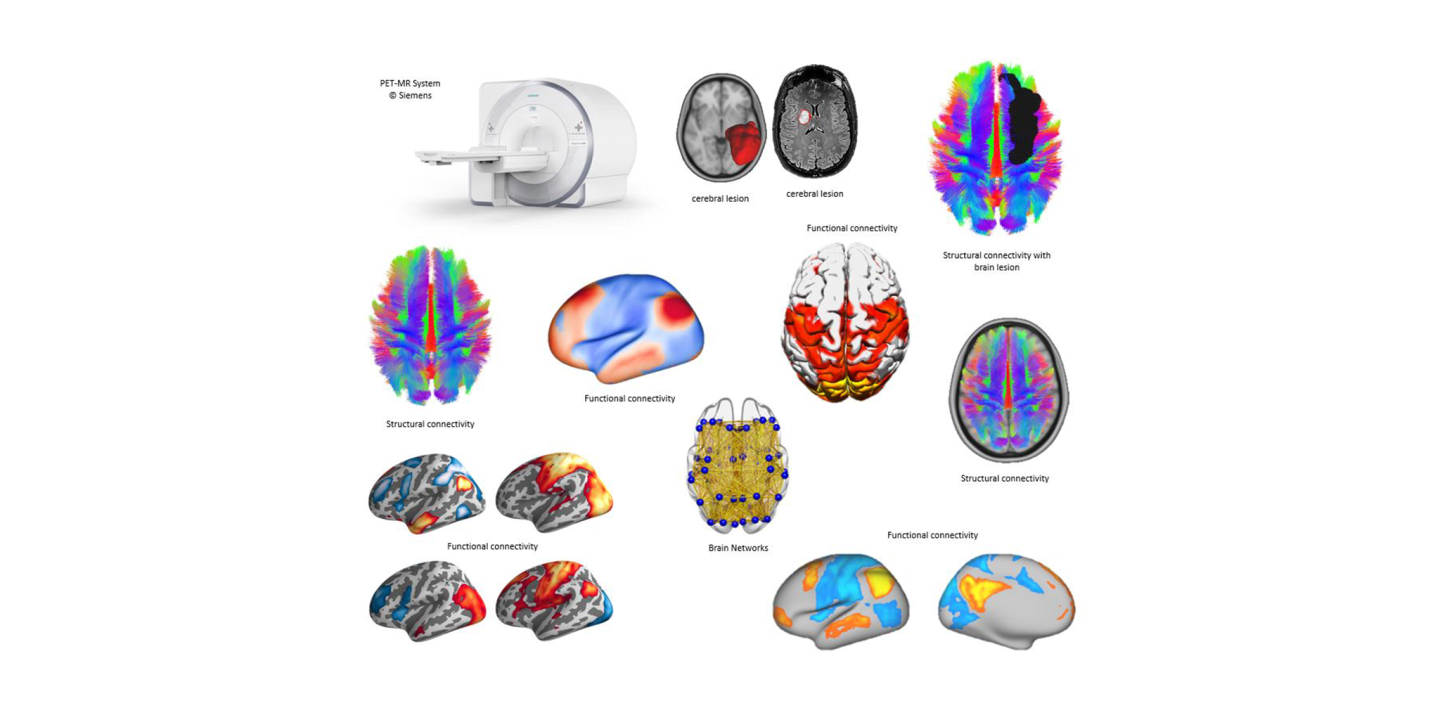
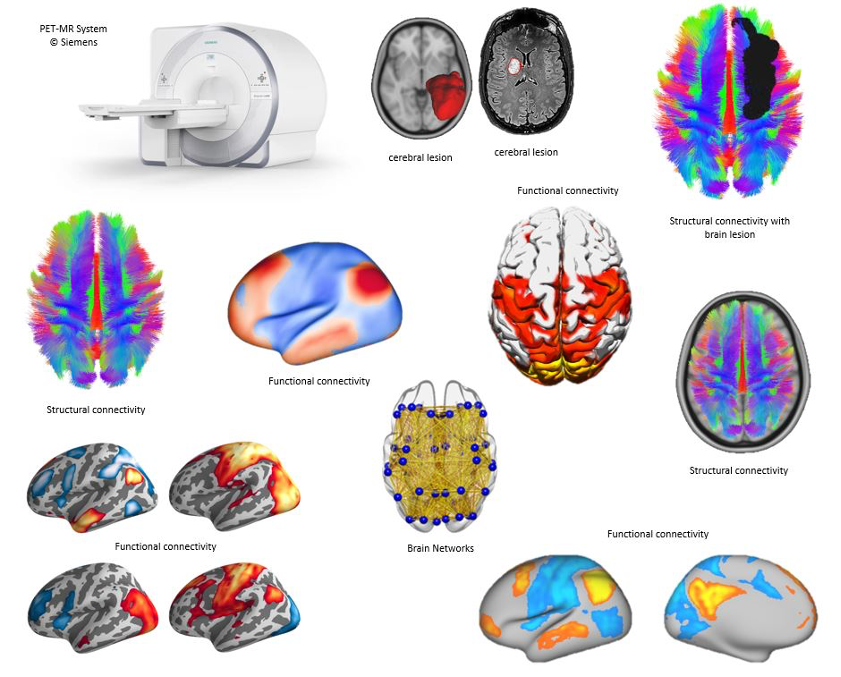
Coordinating a network of 20 neuroscience institutes across Europe, Corbetta’s Work Package in EBRAINS 2.0 has two ambitious goals: collecting a large clinical dataset and employing a special hybrid imaging scanner for an unprecedented type of multimodal brain imaging in heathy individuals. In Padua, the scanner is in the Nuclear Medicine Unit directed by Prof. Diego Cecchin of the Department of Medicine – DIMED.

“EBRAINS’ WP2 is all about using neuroimaging to further connect between the brain atlases and modelling and simulation on EBRAINS” explains Corbetta. The main focus is feature extraction: large amounts of data can be analysed to identify differences and commonalities between brain scans. “We could more precisely pinpoint correlates of damaged areas of the brain or different pathologies. And this base research builds the foundation for clinical applications”.
In particular, the new multimodal dataset (nicknamed the 5M connectome), expected to be completed in 30 months from now, would be novel in approach and unparalleled in magnitude. To achieve this, the project plans to scan 200 healthy subjects of different ages, using five imaging modalities all at the same time (Positron Emission Tomography, Structural Magnetic Resonance, Functional Magnetic Resonance, Diffusion Tensor/Diffusion Weighted Tractography, and high-definition Electroencephalography. Machines capable of this are rare – Munich, Vienna and Copenhagen are the other 5M connectome university centres in the EBRAINS 2.0 project and will be centrally coordinated by Padua.

The substantial amount of data will then provide a baseline for normal brain performance and feature extractions. Alessandra Bertoldo, full professor of Bioengineering at the University of Padua and Director of the PNC co-leads the 5M-connectome project and coordinates the four centers. “This could become a fundamental dataset, similar to ones like 1000Brains, fuelling research for years to come” says Bertoldo.
“The dream would be to then turn all this data into a sort of equation where we can input glucose consumption, cortical thickness, tract density, functional connectivity, and EEG signals to predict cortical and subcortical individual variability. This data would be invaluable when integrated within high resolution atlases already existing in EBRAINS” explains Corbetta. “It would also provide those who work in simulation with precise, multiscale info for the different brain areas. These data are expected to greatly enhance diagnostic prediction, to be able to predict, for example, if a patient with a specific lesion in a part of the brain is likely to improve in the next 6 to 12 months, or if the affected area is suitable for neuromodulation treatments”.
Datasets are more and more prominent and accessible in research, but the problem remains their compatibility. “Common protocols are hard to establish,” says Corbetta. “Every lab does its thing, and data ends up not being comparable”. A member of his team, Lorenzo Pini, is working to address this – a toolbox, working title Actio, which will be available in EBRAINS, that harmonises existing data and makes it legible across datasets. “I took the main features and algorithms of the most used imaging softwares in our field, worked out similarities, and established a bottleneck for elaborating data in a similar fashion” Pini explains. “We are now working on shared guidelines for the EBRAINS community”.
While WP2 aims to establish databases with increased order of magnitude compared to currently available ones, clinical translation remains a key aspect. “We will focus on three case studies, the first one being determining outcomes for stroke.” says Corbetta. “Then there is glioma. Every other type of tumour, except brain tumours, has seen an increase in patient survival in the past 20 years. We recently found out that the inherent connectivity of brain networks may be the explanation: tumour cells interface with healthy ones to protect their survival and spread across the brain. We need to begin considering brain tumours as interacting with the brain, and not extraneous bodies”.
As his research team outlines in a recent paper published in JAMA Neurology, and a recent review in Lancet Neurology, the densest tracts of white matter become preferential paths, almost like train tracks, for the tumour to spread around the brain. “We found out that we could predict the outcome of the tumour by looking at the connectivity of the affected area” says Alessandro Salvalaggio, part of Corbetta’s team and lead author of the study. “And we can do that by looking at already existing scans of the patient”.

The third case study is characterising Parkinson’s disease. “We still don’t know well enough what it is” says Corbetta. “It is a diversified condition that likely includes different groups of patients. By comparing data across the EBRAINS centres, we can identify different subcategories, and establish a much clearer diagnosis and prognosis of this very common disorder”. Like with all big database endeavours, the goal is to make it as open as possible, concludes Corbetta right before attending an online seminar aimed at young European neuroscientists. “We want this data to be available, to draw in as many people as possible – and in particular young researchers. We would like to offer tools and data for generating new research in clinical neuroscience for years to come”.
Further information
Maurizio Corbetta at the EAN congress 2024, Helsinki
Tuesday, 2 Jul, 10:35 - 11:05 EEST
Talk abstract
8th BigBrain workshop, Padua, Italy
September 10 and 11, 2024
HIBALL Lecture in Brain Analytics and Learning
Reference:
White Matter Tract Density Index Prediction Model of Overall Survival in Glioblastoma, Alessandro Salvalaggio et al, JAMA Neurology, DOI: 10.1001/jamaneurol.2023.3284
Glioblastoma and brain connectivity: the need for a paradigm shift
Alessandro Salvalaggio, Lorenzo Pini, Alessandra Bertoldo, Maurizio Corbetta
https://www.thelancet.com/pdfs/journals/laneur/PIIS1474-4422(24)00160-1.pdf
Text by Roberto Inchingolo
Create an account
EBRAINS is open and free. Sign up now for complete access to our tools and services.